***********************************
Brakke Consulting’s
Animal Health News & Notes for September 2, 2022
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Apiam Animal Health
Chewy
ECO Animal Health
Kane Biotech
Patterson
Other News
Asteris
Bactolife
Dechra
DW Healthcare Partners
Med-Pharmex
Merck
MiAlgae
Patterson Veterinary
Petco
Pharmgate
***************************************
K-STATE OLATHE
Animal Health Workshops and Seminars
Upcoming Events
Options available for both online and in-person attendance.
Deviations, Root Cause Investigations and CAPA | Sept. 15
Join us for a review of CVB and CVM requirements for deviations, investigations, root cause analysis and corrective actions. This hands-on discussion will give you the skills you need to navigate the requests of the FDA and USDA.
Pesticides in Animal Health | Oct. 12-13
The workshop will provide a practical approach to getting pesticide products approved and keeping them in the market post approval, while avoiding common pitfalls and challenges faced along the way. The workshop is designed to be interactive with case studies, and an opportunity for participant interaction and networking.
Leadership Program for STEM Professionals | Begins Sept. 22
Take your management abilities to the next level with K-State Olathe’s leadership program that provides the tools and skills you need to succeed. Strategically designed to build upon itself with each workshop, this series will help you achieve the results you and your company aspire to. Discover your own strengths and communications style, learn conflict resolution skills, understand how to connect with your team across differences, and develop future-focused strategies to achieve lasting results.
Click on the seminar name for cost and registration details.
**************************************
EARNINGS NEWS RELEASES
- Patterson Companies, Inc. reported financial results for its fiscal first quarter ended July 30, 2022. Reported net sales in the Animal Health segment were $960 million, representing internal sales growth of 5.7% year-over-year. Animal Health segment operating income was $21.9 million, a decline of 8%. (company press release)
- Chewy, Inc. reported financial results for the second quarter of fiscal year 2022 ended July 31, 2022. Net sales were $2.43 billion, an increase of 13% year-over-year. Net income was $22.3 million compared to a net loss of $(16.7) million in the prior-year period. (company press release)
- ECO Animal Health reported final results for the year ended 31 March 2022. Group sales were down 22% to GBP 82.2 million ($96 million). Net loss for the year was GBP(705,000) ($824,000) compared to net income of GBP 15.8 million in fiscal 2021. (company press release)
- Apiam Animal Health Limited announced its full year results ended June 30, 2022 (FY22). Revenue was A$157 million (US$108 million), an increase of 25% driven by growth in Apiam’s dairy and mixed animal segment. Underlying Net Profit After Tax (NPAT) increased 15% in FY22 to A$6.7 million (US$4.6 million). (abnnewswire)
- Kane Biotech announced its second quarter 2022 financial results. Total revenue was C$839,579 (US$649,053), an increase of 201% compared to the quarter ended June 30, 2021. Loss was C$(794,595), a decrease of 26% compared to the prior-year quarter. (company press release)
*********************************************
COMPANY NEWS RELEASES
- The FDA announced a supplemental approval for Merck’s Bravecto and Bravecto Plus, which provides for the addition of the indication for the treatment and control of Haemaphysalis longicornis (Asian longhorned tick) infestations for 2 months in cats and kittens. (FDA)
- Dechra Pharmaceuticals announced it has acquired Med-Pharmex from DW Healthcare Partners for $260 million. Med-Pharmex generated audited revenues of $43 million and an adjusted EBITDA of $15.3 million in 2021. (Sharecast, PRnewswire)
- The FDA announced approval of Pharmgate‘s ANADA for Pennitracin MD and Coban (bacitracin and monensin Type A medicated articles) in poultry for the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens, and as an aid in the prevention of coccidiosis. (FDA)
- Petco Health and Wellness Company, Inc. announced the expansion of Vital Care, its paid health and wellness membership program, to now include birds, reptiles, fish and small pets. Previously Vital Care was only available for dogs and cats. (company press release)
- Patterson Veterinary and Asteris announced an integration between NaVetor veterinary cloud software and Asteris’ Keystone PACS system, which will allow NaVetor to connect with almost any modality-work-list-enabled digital x-ray system and provides immediate and secure access to image files at any time, from any location. Veterinarians can access their diagnostic-quality images right from within NaVetor’s patient record. (company press release)
- SCOTLAND Edinburgh-based MiAlgae closed a round of GBP 2.3 million ($2.7 million), which will help the company to build a commercial demonstrator plant by 2023. MiAlgae has developed a novel biotechnology platform that uses low-value co-products from the food and drink industry as a feedstock to initially grow microalgae rich in Omega-3 oils. The company is focused on the pet food sector with an eye on expansion into the aquaculture sector in the near future. (Globalpets)
- DENMARK Bactolife announced it has received $5 million in funding from the Bill & Melinda Gates Foundation to address gastrointestinal infections. Bactolife has three active projects in animal health; its lead program is Ablacto+, a feed additive comprising Binding Proteins that reduces the risk of enterotoxigenic Escherichia coli-related post-weaning diarrhea. (IHS Markit Connect – subscription)
*********************************************
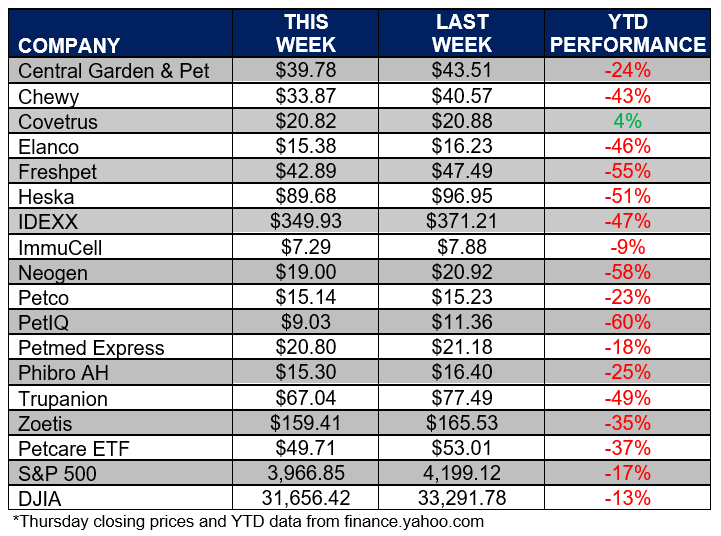
ANIMAL HEALTH STOCK PRICE TRACKER
***********************************
ANIMAL HEALTH NEWS
- VIETNAM – ASF Vietnam has suspended use of its first African swine fever vaccine after dozens of pigs inoculated with the shots died this month. The pigs were among about 600 pigs at several farms that were injected with the NAVET-ASFVAC vaccine developed by Navetco, a company owned by the agriculture ministry. Vietnam in June announced it had successfully developed a vaccine to administer to pigs to fight ASF, with the aim of becoming the first country to commercially produce and export it. (Vet Advantage)
- US – RABIES PREVENTION The USDA has begun scattering millions of packets of oral rabies vaccine from helicopters and planes over 13 states from Maine to Alabama, with a goal of keeping raccoons from spreading their strain of the virus to states where it hasn’t been found or isn’t widespread. The vaccine has been found safe for more than 60 kinds of animals including domestic dogs and cats. The USDA is also continuing tests of a vaccine approved in Canada to immunize skunks as well as raccoons. (AP)
- US – FDA The FDA’s Center for Veterinary Medicine announced that registration is now open for a virtual public listening session on October 18, 2022 beginning at 10:00 a.m. EDT on the Agency’s regulation of animal foods with certain types of claims such as environmental benefit claims (e.g., reduced greenhouse emissions), production claims (e.g., growth promotion, feed efficiency), and claims about effects on the animal microbiome. The FDA is reviewing CVM Policy and Procedures Manual (PPM) 1240.3605, Regulating Animal Foods with Drug Claims, and is seeking public comments on how the existing policy could be updated to reflect evolving scientific knowledge and promote innovation. Stakeholders interested in attending the virtual listening session should register no later than October 11, 2022, at 11:59 p.m. ET. In addition to holding the listening session, the FDA is accepting electronic or written comments through November 17, 2022. To electronically submit comments to the docket, visit gov and type “FDA-2022-N-2015” in the search box. (FDA)
- US – FIP GUIDELINES The American Association of Feline Practitioners (AAFP) and EveryCat Health Foundation have released the 2022 AAFP/EveryCat Feline Infectious Peritonitis Diagnosis Guidelines. These landmark Guidelines are published in the Journal of Feline Medicine and Surgery and will provide veterinarians with the essential information necessary to provide a FIP diagnosis in cats. (association press release)
************************************
BRAKKE CONSULTING VIEWPOINT
This week found me thinking more about innovation in the animal health industry, primarily driven by attending the 2022 Animal Health Corridor Summit on Monday and Tuesday. KCAHC’s Kim Young and Emily McVey did a great job organizing a sold-out event that had a theme of brining innovation to our industry.
One half day of the Summit was dedicated to presentations from emerging companies, and I caught up with Tom Overbay of Expedite Animal Health, who led the coaching and selection teams for these start-ups, to find out more about them. There were 32 emerging companies that applied to present at the Summit and of these, surprisingly, about 60% of them were focused on livestock and 40% on companion animals. And of these 32, about 15% were product related, where an FDA/USDA approval would be needed and 85% were diagnostic or technology related. Of the 12 finalist companies, 7 were diagnostic or technology related, and an emerging diagnostic company won the competition, Vidium Animal Health.
Fabian Kausche touched on this at the Summit in his presentation, titled “Transitions in Innovation in Animal Health” where he reminded us of the historical waves of innovation in animal health – we are in a technology-based wave of innovation. But I cannot help but think expensive FDA user fees hamper drug innovation, a topic raised in a recent Viewpoint.
If you lead or know someone in an emerging company or would like to voice your opinion on FDA user fees and innovation, you have a chance to do so on September 6th from 10-11 am. Click here to join the meeting, where the FDA will be in “listening mode”. These sessions are generally very poorly attended. Written comments may be also submitted to the docket at any time by clicking here and currently, there are very few comments posted. Innovation in animal health is not easy, but perhaps if the FDA hears from you, they can help make new drug approvals easier.
Bob Jones
***********************************
YOUR VIEW
Last week we asked about VCA’s new Urgent Care veterinary service model from a business standpoint, and 22% thought it was a home run. Over 66% thought it was either promising or it has potential. So, our readers are rather positive on the concept.
