***********************************
Brakke Consulting’s
Animal Health News & Notes for July 29, 2022
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Brakke Consulting News
Cancer in Dogs and Cats – pre-order now
Earnings News
Animalcare
Krka
Merck
Neogen
PetMed Express
Other News
Animol Discovery
Anterra Capital
BASF
Biogénesis Bagó
Bionote USA
Cargill
Central States Enterprises
Continental Grain Company
Family Vet Group
Furst-McNess Company
FVC
Heartland Veterinary Partners
Incline Equity Partners
Mars Petcare
Mountain Group Partners
Novalis Lifesciences
PSIvet
QBiotics Group
Revival Animal Health
Sanderson Farms
Verility
Wayne Farms
Wayne-Sanderson Farms
Wedgewood Pharmacy
*********************************************
2022 ANIMAL HEALTH SUMMIT
August 29-30, 2022
Location: Arvest Bank Theatre at the Midland | Kansas City, MO
Registration is now open for the 2022 Animal Health Summit.
Register today! The Animal Health Summit features two days of networking, 1:1 business partnering, industry thought leaders, panel discussions, speakers and emerging company presentations. Learn more.
************************************
EARNINGS NEWS RELEASES
- Merck announced financial results for the second quarter of 2022. Animal Health revenues were $1,467 million, unchanged from the prior-year quarter (+5% currency adjusted). (company press release)
- Neogen Corporation announced the results of its full 2022 fiscal year ended May 31. Revenues were $527 million, an increase of 13% over the prior year. Net income was $48.3 million; excluding $25.6 million in 3M deal-related costs, net income would have been $67.9 million, an overall year-over-year increase of 12%. The Animal Safety segment recorded revenues of $267 million, an increase of 14% over the prior year (12% organic growth). (company website)
- PetMed Express, Inc. announced its financial results for its first quarter ended June 30, 2022. Net sales were $70 million, a decrease of 11.5% compared to the prior year quarter. Net income was $2.8 million, compared to net income of $4.4 million for the prior year quarter. (Globenewswire)
- Animalcare Group reported results for the first half of 2022. Sales totaled GBP 38.3 million ($46 million), a decrease of 2% compared to the first half of 2021. (TheBusinessDesk)
- Krka reported results for the first half of 2022. Animal health revenues were EUR 47.2 million ($47.9 million), a year-on-year increase of 5%. (IHS Markit Connect – subscription)
*********************************************
BRAKKE CONSULTING
2022 CANCER IN DOGS AND CATS REPORT – COMING IN SEPTEMBER
EARLY ORDER DISCOUNT NOW THROUGH AUGUST 26

Did you know that cancer is the #1 cause of death in adult dogs? It’s also a leading health concern of dog owners. So how is cancer addressed by the veterinary profession, and what kind of market is there for veterinary cancer therapies?
Brakke Consulting’s new 2022 Cancer in Dogs and Cats report includes information on the incidence, diagnosis, and treatment of the common veterinary cancers. It covers currently approved veterinary therapies and those in development, as well as estimates of the numbers of pets treated.
The study includes a survey of 300 general veterinary practitioners about incidence of cancer and frequency of treatment; as well as a survey of 1,000 pet owners to determine what they are willing to pay to treat a pet with cancer.
The report is available for pre-order for $7,995 until August 26, and will be published in September; after August 26 the price will be $8,500. For more information, please contact Dr. Lynn Fondon at lfondon@brakkeconsulting.com.
*********************************************
COMPANY NEWS RELEASES
- QBiotics Group Limited announced it has been granted approval of its Investigational New Drug (IND) application by the US FDA for tigilanol tiglate to initiate a Phase II clinical trial in patients with soft tissue sarcoma. Tigilanol tiglate is currently FDA approved as Stelfonta for the treatment of mast cell tumors in dogs. (PRNewswire)
- Wedgewood Pharmacy announced that the New York State regulatory authorities have issued a license to Wedgewood Connect – the FDA-registered 503(B) Outsourcing Facility owned by Wedgewood Pharmacy – to offer compounded medications for in-office use to veterinarians in New York. (company press release)
- Bionote USA announced a new partnership with PSIvet to make Bionote USA’s Vcheck line of quantitative, point-of-care veterinary analyzers and tests more affordable to PSIvet’s 5,000-plus veterinary practices. (company press release)
- Revival Animal Health and Incline Equity Partners announced Lynn Snodgrass as Revival’s new President & CEO, replacing retiring President & CEO Jim Rossiter. (company press release)
- Mars Petcare announced that it has created a quality of life assessment that evaluates dog health and wellbeing. Using data points from 2,813 dogs, the research team put together an assessment framework based on a 32-item questionnaire for owners to report their dog’s behavior and activity. A paper published in Nature’s Scientific Reports supported the validity of this assessment for measuring and quantifying canine health and wellbeing. The assessment’s validity was examined by comparing the owner survey results with Banfield medical records of the dogs studied. (IHS Markit Connect – subscription)
- Verility – creator of a global platform called Fertile-Eyez that provides fertility analysis products that enable livestock producers and breeders to accelerate reproductive performance – announced it has closed Series A funding worth $3.5 million. The round was led by Mountain Group Partners. (Feedstuffs – subscription)
- Animol Discovery, announced it has raised $34.0 million in Series B financing. The investment was led by Novalis Lifesciences, with participation from existing investor Anterra Capital. Animol is using DNA Encoded Library (DEL) drug discovery technology coupled with machine learning approaches to enable the discovery of novel veterinary medicines. (Businesswire)
- Heartland Veterinary Partners announced that it has acquired Family Vet Group, providers of general practice veterinary care with locations in major metropolitan areas across the South and Southeast. Terms of the deal were not disclosed. (PRnewswire)
- BASF announced it is combining its human and animal nutrition units into one global business unit. The new division – Nutrition Ingredients – will allow BASF to have one unit focused on supplying both the animal nutrition and human nutrition industries, enabling it to leverage large-scale product platforms for vitamins and carotenoids, as well as expand its feed enzymes business. BASF will continue to serve its customers in animal nutrition and human nutrition with dedicated teams. (IHS Markit Connect – subscription)
- Cargill and Continental Grain Company announced the completion of the previously announced acquisition of Sanderson Farms by a joint venture. As a part of the closing of the transaction, Sanderson Farms will be combined with Wayne Farms, a subsidiary of Continental Grain, forming a new privately held poultry business named Wayne-Sanderson Farms. Sanderson Farms shareholders are receiving $203.00 per each share of common stock they owned as of immediately prior to the completion of the $4.53 billion transaction. (Feedstuffs – subscription)
- Furst-McNess Company – a provider of commodities, blended feeds, and nutritional products to dairy, beef, and poultry producers in Florida and Georgia for over 30 years – announced it has acquired manufacturing and transloading facilities as well as a retail storefront in Florida, previously operated by Central States Enterprises, LLC. Per the terms of the transaction, McNess will continue to offer CSE branded products. (WattAgnet)
- SOUTH KOREA Biogénesis Bagó announced its consortium with the South Korean company FVC for the construction of a world class site in the Korean town of Osong that will produce up to 100 million doses of Foot and Mouth Disease vaccine per year. The project will require an investment of around $50 million and is expected to be completed in 2023. (Streetinsider)
*********************************************
ANIMAL HEALTH STOCK PRICE TRACKER
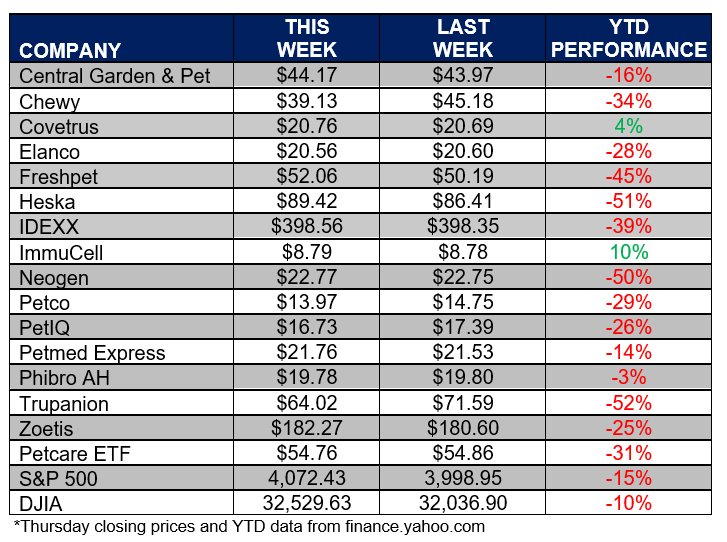
***********************************
ANIMAL HEALTH NEWS
- US – DRUG USER FEES The FDA announced the fiscal year (FY) 2023 fee rates and payment procedures for animal drugs subject to user fees under the Animal Drug User Fee Amendments of 2018 (ADUFA IV) and Animal Generic Drug User Fee Amendments of 2018 (AGDUFA III). Click here for details. (FDA)
- US – DRUG GUIDANCE The FDA has withdrawn guidance for industry (GFI) #210, “The Index of Legally Marketed Unapproved New Animal Drugs for Minor Species” in order to revise it in accordance with a statutory change to the wording of required label statements and because the guidance no longer represents the agency’s current thinking. The FDA plans to issue a revised draft guidance as expeditiously as possible. (FDA)
- US – VETERINARY SHORTAGE The American Association of Veterinary Medical Colleges has issued a statement on the U.S. veterinary workforce shortage. “Significant shortages of veterinarians exist across all sectors of professional activity and at all levels of specialization,” the AAVMC says. “Although precise numbers are difficult to quantify and specific predictions about future needs are subject to interpretation, the available evidence indicates that these shortages are a result of systemic, long-term trends in pet ownership and demand for veterinary services, along with limited capacity for training veterinary professionals, and are expected to continue unless the veterinary medical profession takes action.” Read the full statement here. (Vet Advantage)
- UK – ANTIBIOTIC RESISTANCE Research at the University of Bristol Veterinary School has shown that dogs who were fed raw meat were more likely to shed antibiotic-resistant Escherichia coli in their feces. The two latest studies researched raw meat diets in both adult dogs and 16-week-old puppies, a total of more than 800 dogs. The team found that dogs who were fed raw meat diets were more likely to excrete antibiotic-resistant bacteria, regardless of age or the length of time they were fed the diet. The findings from the second study were published July 21 in the Journal of Antimicrobial Chemotherapy, while the other report was published earlier this year in the journal One Health. (AVMA SmartBrief – Sciencedirect)
************************************
BRAKKE CONSULTING VIEWPOINT
Do high FDA Animal Drug Users Fees slow innovation in animal drugs?
Each year the FDA ADUFA/AGDUFA fees change due to inflation, workload, and prior year excesses or shortfalls in collections. Starting in October, New Animal Drug Applications will require a $659,364 check and a supplemental application will need a $329,682 check, 13.5% more than last year. A generic drug ANADA will need a $494,983 check in 2023, down from $548,628 in 2022 due primarily to excess collections in 2021. That’s a lot of money and it must impact innovation.
Some interesting information on innovation comes from the workload calculation. The average number of NADAs submitted over the last 5 years (ending May 2022) is 12.8, which is down 22% compared to the 5 years ending September 2018, which had an average of 16.4 applications. The FDA expects 5.25 NADAs in 2023. Comparing these same 5-year periods to each other, supplemental applications are down 22% also, investigational study submissions are down 7%, manufacturing supplements are up 4% and investigational protocol submissions are up 1%. This all leads to an overall 4.5% reduction in workload, which doesn’t reflect well on innovation.
However, the workload for Generic Animal Drugs is booming – it’s up 77%. Over these same two 5-year periods, ANADAs are up 12.5% (27 vs 24), generic investigational study submissions are up 125%, generic investigational protocol submissions are 77% and manufacturing supplements are up 30%. The FDA estimates that in 2023, there will be 11.6 full fee ANADAs filed, double the NADAs filed.
So, less risky, cheaper generic drug development has replaced more risky, expensive new drug development. There are provisions for fees to be waived or reduced if the product is deemed to be innovative and that the fee would be a significant barrier to bringing the product to market. The number of NADAs that were filed with the FDA and had the fees waived is not published by the FDA – we assume it is insignificant.
This is the last year of ADUFA IV and negotiations are underway now for ADUFA V, which would begin in FY2024. Let’s hope its structured to drive more innovation.
Bob Jones
***********************************
YOUR VIEW
Last week, we asked about work/life balance. 45% of you said that in the past year you have partially refocused and redirected, with an emphasis on your life balance; and 12% have partially refocused with an emphasis on a new area of work. 16% have completely refocused and redirected your careers, and 27% say your work/life aspirations have remained unchanged.
This week
