***********************************
Brakke Consulting’s
Animal Health News & Notes for June 25, 2021
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Patterson Vet
Other News
Algenex SL
Animalcare
Antelligence
Axxiom Training Solutions
BRF SA
Chasing Our Tails
Chewy
Covetrus
CUBEX
DCN Dx
Dechra (Porus)
Dechra (Intervacc)
Diggs
DSM
FATRO S.p.A
Fold Hills Foods
Freshpet
Hercosul
Hill’s
Intervacc
Just Kitchen Holdings
Kinship
Mars
Marsh Dog
Novozymes
Orion Animal Health
PetDesk
PortaScience
Porus GmbH
venn growth partners
************************************
Southwest Veterinary Symposium – FURther Together
SWVS 2021 will be LIVE and IN-PERSON!
San Antonio, Texas – Henry B. Gonzalez Convention Center
September 23-26, 2021
Early Bird Registration — Now Until July 15th
Registration is NOW OPEN! Southwest Veterinary Symposium is a partnership of the Arkansas, Louisiana, New Mexico, Oklahoma and Texas Veterinary Medicine Associations which provides continuing education for veterinary professionals. Join us live in-person this September in San Antonio, Texas, where you can learn about the latest innovations and products while exploring our exhibit hall, network with other veterinary professionals, and choose from more than 400+ hours of quality CE/RACE approved sessions.
************************************
COMPANY EARNINGS RELEASES
- Patterson Companies reported financial results for its fiscal year ended April 24, 2021. Net sales in the Animal Health segment were $3.56 billion, an increase of 7% over the prior year (+6% currency adjusted). Operating income for the segment was $88.1 million compared to an operating loss of $(595) million in fiscal 2020. (company press release)
************************************
COMPANY NEWS RELEASES
- Dechra Veterinary Products announced a sales and marketing agreement with Porus GmbH for Porus One, a supplement recommended for the support of feline kidney health in the US and Canada. This will be Dechra’s first renal health product for cats. (company press release)
- Hill’s Pet Nutrition announced plans to invest more than $250 million to build a new manufacturing facility in Kansas. (PRNewswire)
- DCN Dx announced it has completed its acquisition of PortaScience, Inc. The acquisition includes the PortaCheck brand of products for animal health management. Financial terms were not disclosed. (PRNewswire)
- Chewy announced it is entering the fresh and prepared pet food space with its premium proprietary brand, Tylee’s. Chewy developed a patented sustainable packaging that allows the company to preserve product quality through the delivery process. Chewy will also offer Freshpet The online retailer will soon launch initial distribution in three geographies covering approximately 60% of its customer base. (Pet Product News)
- The DSM-Novozymes Feed Enzymes alliance announced the commercial release of its second-generation protease, ProAct 360 for poultry. (Fareasternagriculture.com)
- Chasing Our Tails announced it has acquired Marsh Dog, a privately-held dog treat firm focused on utilizing the protein of wild nutria. Chasing Our Tails will be re-launching Marsh Dog’s current line-up later this month with line extensions to follow. Financial terms were not disclosed. (Pet Business)
- Just Kitchen Holdings announced that it has recently launched its proprietary Wow Chow brand and associated menu of fresh, delivery-only pet food. Wow Chow items are currently available to customers through Uber Eats and the Company’s JustMarket e-commerce website in Taiwan. Wow Chow items are currently available to customers through Uber Eats and the Company’s JustMarket e-commerce website in Taiwan. (Yahoo finance)
- Diggs – maker of the Revol Dog Crate – announced it has closed a $13 Million Series A funding round. The investment round was led by venn growth partners. (GlobalPets)
- CUBEX announced an agreement for Covetrus to become an authorized reseller for CUBEX solutions. Covetrus will also offer attractive financing and rebate programs on CUBEX that are not available elsewhere. (Yahoo finance)
- Veterinary Hospitals Association (VHA) announced a partnership agreement with PetDesk to offer VHA members PetDesk’s suite of tools to help improve communication flows, reminders, and appointment requests. (grandrapidsmn.com)
- Mars subsidiary Kinship announced it has partnered with Shelter Animals Count (SAC) to improve the latter’s measurement and reporting capabilities. SAC is an independent non-profit organization that is home to a national database of sheltered animal statistics. (IHS Markit Connect)
- Antelligence, LLC announced it has partnered with Axxiom Training Solutions (ATS) to release an updated version of its Foundations VIII Introduction to Animal Health Industry Training Program, designed for individuals who are relatively new to animal health, typically with a year or less of experience, who will benefit from comprehensive overview the entire industry. (company press release)
- EU Orion Animal Health announced that the European Medicines Agency’s Committee for Medical Products for Veterinary Use (CVMP) has recommended Tessie (tasipimidine) for marketing authorization in the European Union. The compound – a selective alpha-2A adrenoceptor agonist – is indicated for short term alleviation of situational anxiety and fear in dogs triggered by noise or owner departure. (Globenewswire)
- EU Animalcare announced the launch of Daxocox (enflicoxib), a selective COX-2 inhibitor and the first and only weekly oral non-steroidal anti-inflammatory drug (NSAID) available to treat osteoarthritis in dogs. (IHS Markit Connect)
- EU Intervacc announced that the European Medicines Agency’s Committee for Medicinal Products for Veterinary Use (CVMP) has recommended Strangvac strangles vaccine for marketing authorization. Intervacc also anticipates receiving a positive opinion for Strangvac in the UK before the end of July. Dechra Pharmaceuticals will be the exclusive distributor of Strangvac throughout Europe once the vaccine is approved; Intervacc will market Strangvac directly in the Nordic and Baltic countries. (IHS Markit Connect)
- EU Algenex SL and FATRO S.p.A announced that the European Medicines Agency’s Committee for Medical Products for Veterinary Use (CVMP) has recommended FATRO’S CrisBio-based vaccine for Rabbit Haemorrhagic Disease (RHD) for marketing authorization in the European Union. (Globenewswire)
- BRAZIL Brazilian food processor BRF SA has reached an agreement to acquire Brazilian pet food company Hercosul for an undisclosed amount. (GlobalPets)
- UK The UK’s Royal Veterinary College (RCV) is investigating an uptick in cats experiencing symptoms of pancytopenia, a blood cell deficiency condition, which has been linked to hypoallergenic cat food produced by Fold Hills Foods Fold Hills Foods issued a recall on June 15 for certain brands of its hypoallergenic cat food products. It is still unclear whether the increased rate of pancytopenia in cats is directly related to the hypoallergenic cat foods listed in the recall. (Petfoodprocessing.com)
***********************************
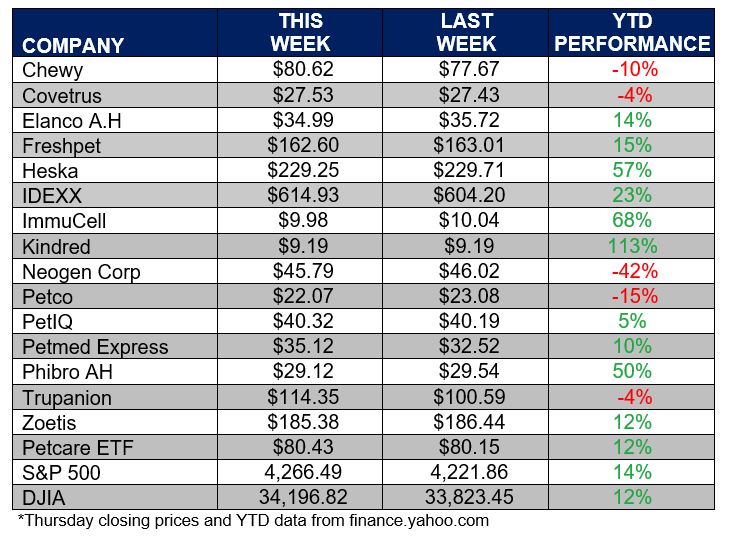
ANIMAL HEALTH STOCK PRICE TRACKER
***********************************
ANIMAL HEALTH NEWS
- US – CBD USE Nevada has become the first US state to legally enable veterinarians to administer cannabidiol (CBD) products to pets or recommend such products to owners. A new bill allows licensed vets to administer products containing hemp or CBD, at a concentration of not more than 0.3% THC, to animals, and authorizes vets to recommend the use of a product containing hemp or CBD to owners to treat an animal’s condition. Additionally, the bill prohibits the Nevada State Board of Veterinary Medical Examiners from taking disciplinary action against a licensed veterinarian for recommending the use of or administering certain products containing hemp or CBD to treat an animal’s condition. The law will take effect October 1, 2021. (IHS Markit Connect)
- US – UNAPPROVED DRUGS The FDA is requesting comments on the agency’s current policy for determining the eligibility of unapproved animal drugs to be added to the Index of Legally Marketed Unapproved New Animal Drugs for Minor Species (the Index). The FDA is asking for public comment on three specific questions: (1) What are the reasons we should or should not expand eligibility for indexing to certain discrete subsets of food-producing minor species? (2) If you support the expansion of indexing, please describe the information we should evaluate when determining which discrete subsets of food-producing minor species should be eligible. (3) Are there any discrete subsets of food-producing minor species that you believe should be eligible for indexing because they are not intended for consumption by humans or food-producing animals? The FDA is accepting public comments 90 days beginning on June 24, 2021. To electronically submit comments to the docket, visit regulations.gov and type FDA-2017-D-2462 in the search box. (FDA)
- US – POULTRY RESEARCH Researchers at the USDA’s Agricultural Research Service and US Biologic Inc. have developed an oral solution to an antibiotic alternative that fights against poultry coccidiosis. The oral product – known as cNK-2 – is delivered in a probiotic powder that can be mixed into current feed processes and then fed to the birds across their lifetime. The USDA-ARS and US Biologic have patented the technology, and US Biologic has signed an exclusive global commercialization agreement with the goal of developing and licensing the technology for industry use. The research was published in the June issue of Frontiers in Veterinary Science. (Feedstuffs)
***********************************
BRAKKE CONSULTING VIEWPOINT
The question we asked our readers last week was partly inspired by the announced acquisition of Kindred Bio by Elanco, which freshens Elanco’s R&D pipeline with a stable of monoclonal antibodies (MAbs) for use in pets. And, we were inspired by the success of Zoetis’s Cytopoint, a MAb for use in dogs with allergic or atopic dermatitis.
The question we asked was, “In five years’ time, what share will all MAbs take of the total osteoarthritis pain market for dogs?” About 20% of respondents said that it was too early to tell, and less than 10% said that MAbs would take less than 10% share. Almost 30% said that MAbs would take 25-50%, with about 20% each estimating 10-25% and 50-75% of the total share. It might be hard to pick a number, but it looks like our respondents see big share changes ahead. So do we.
It is hard to overstate the importance of MAbs in human medicine since the first product was introduced in 1975. Estimates of the current global market size for human MAbs range around $125B (about half of that is in North America), with growth rates expected to be between 7 and nearly 15% per year over the next few years. Given the pipelines in animal health companies, it will not be too long when we will look back on how MAbs dramatically changed the way veterinarians treat chronic diseases in pets.
Bob Jones
***********************************
BRAKKE CONSULTING VIEWPOINT
There are several news items this week about pet foods– Hill’s is adding manufacturing capacity, Chewy will enter the fresh (refrigerated) pet food space, and a few others. Lets get a read on how our readers view the fresh (refrigerated) pet food market.
For your pets, have you fed them a fresh (refrigerated) pet food diet?
