***********************************
Brakke Consulting’s
Animal Health News & Notes for September 26, 2025
Copyright © Brakke Consulting
Editor: Christine Merle DVM, MBA, CVPM
***********************************
IN THE NEWS:
Brakke Consulting News
Brakke Search – Director of Quality Assurance (USDA)
Company News
AA Biotek ( PHIL)
ADM
Alltech
Dechra (UK)
Elanco
Gallant
Jaguar Health
Lonza
Mars (EU)
Merck (CA)
PetVivo Animal Health
RION
Virbac (EU)
WEDterinary
Zomedica
Animal Health News
Australia
Brazil
Mexico
Russia
United States
***********************************
Brakke Consulting
Director Quality Assurance (USDA)
Are you a QA expert with strong USDA experience? Do you flourish in a small, informal environment where you roll up your sleeves on groundbreaking new projects in a brand new, state-of-the-art facility? Are you attracted to an up-and-coming company that offers a competitive compensation package including equity, and a comfortable but focused work environment? If so, we’d like to talk to you! Our client is hiring now. This is an onsite position located in Shreveport, LA, a great way to make an impact in the company and industry. For more information, contact Jeff Santosuosso
***********************************
Company News Releases
- Based on supplemental scientific data, the FDA provided an update to Elanco’s Zenrelia label, removing the risk of fatal vaccine-induced disease from modified live virus vaccine from the labeling. ( elanco.com)
- Elanco and WEDterinary have entered a partnership to explore ways to use targeted gene therapies to treat chronic kidney disease and extend pets’ lifespan. ( com)
- The addition of feline cobalamin and folate testing to Zomedica’s TRUFORMA diagnostic platform was announced. (com)
- Gallant has received a complete letter for Reasonable Expectation of Effectiveness technical section from the FDA-CVM for it’s stem cell therapy for refractory feline chronic gingivostomatitis. (com)
- Jaguar Animal Health has received a $250,000 grant from the FDA that will fund a confirmatory trial for Canalevia- CA1, their conditionally approved product for chemotherapy- induced diarrhea. ( theglobeandmail.com)
- An agreement between ADM and Alltech will form a North America animal feed joint venture consisting of 29 feed mills in the US and 15 in Canada. (com)
- Three new RACE approved CE offerings have been launched by PetVivo Animal Health, a veterinary medical device company. (yahoo.com)
- Lonza will be providing manufacturing and technical support for the commercial scale production of RION’s platelet-derived exosome for late phase clinical supply and beyond. ( (com)
- CA- Merck launched in Canada Engemycin DD, a new short acting antibioitic for cattle, pigs and sheep. (com)
- UK- Dechra has added a 20mg hard capsule to its Vetoryl (trilostane) line. ( vettimes.com)
- EU-The first medicated feed for cats, Vikaly, was launched in Europe by Virbac. (com)
- EU -Mars announced plans to invest 1 billion euros ($1.18 billion ) in its EU operation including upgrades to its pet food facilities. (net)
- PHIL- A partnership between AA Biotek, a biotechnology company and the Philippine Carabao Center will bring probiotic-based innovation and eco-friendly agriculture to the Phillipines’ farmers and agribusinesses. ( com)
***********************************
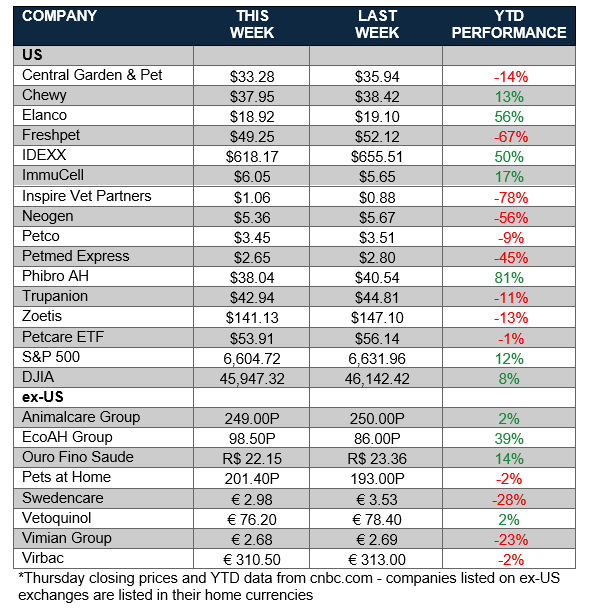
Animal Health Stock Price Tracker
***********************************
Animal Health News
- US- LISTENING SESSIONS- The USDA will hold listening sessions to learn from key stakeholders on Barriers to Entry and Increased Recruitment for Rura and Federal Veterinary Workforces on September 29 and September 30. For more information see news release at usda.gov.
- US- TELEMEDICINE- Two bills signed into law in Arkansas authorized veterinary telemedicine in the state and allows veterinary technicians to go on location for emergency calls without a veterinarian. (com)
- MEXICO- NEW WORLD SCREWWORM- Mexico confirmed a case of New World Screwworm in Sabinas Hidalgo, located less than 70 miles from the US-Mexico border. The location is near the major highway from Monterrey to Laredo, Texas and is one of the most commercial throughfare in the world. ( gov)
- BRAZIL- BIRD FLU- The European Union has recognized Brazil as free from bird flu, and has begun negotiations to resume chicken imports to the EU. ( com)
- RUSSIA- FELINE VACCINE- The All-Russian Scientific Research Institute has released a comprehensive vaccine for cats, Carnifel PCHR, that provides protection against both infectious diseases and rabies in a single vaccine. Vaccination includes two doses and then annual revaccination. ( net)
- AUSTRALIA- AQUACULTURE- The Center for Aquatic Animal Health and Vaccines in Tasmania have a new vaccine designed to treat a Piscirickettsia salmonis (seafoodsource.com)
************************************
Brakke Consulting Viewpoint
I continue to think about the number of innovative products necessary to meet the expected growth of the animal health market over the next 10 years, especially given the generic parasiticides we expect to see on the market for dogs. When taken in the context of the ongoing discussion about reducing the necessity for animal testing in the FDA approval process, how will this impact future development plans?
Some of the expected benefits of less animal testing include:
- Reduced timeline and cost of approval, with some suggesting it could reduce time and/or cost by 20% or more
- Ethics and animal welfare resulting in fewer animals being needed for terminal studies
- Use of computational models and microphysiological systems to facilitate studies
- Overall expedited approval to support market growth
- Facilitate development and investment in medications that might not reach blockbuster status
Each of these comes with the challenge of how to manage any transition:
- Initial investment in technology
- Ensuring the necessary regulatory endpoints can be met from non-animal studies
- Having proven, reliable and consistent processes that are validated
- Accurate alternative methods that correctly quantify risk
- Chaos of living through a change
There is no clear guidance for the animal health industry and most suspect this will be dealt with on the human side first. It is impossible to eliminate all animal studies for veterinary products. I think we can agree that adoption of new methods that can decrease the approval timelines and costs while ensuring the continued safety and efficacy of the medications will be good for future innovation.
Chuck Johnson
***********************************
Your View
Last week we asked Which is the last segment of horse owners? 27% selected the 18–34 age group, 37% chose 35–54, and 36% said 55 and older.
The correct answer is 55+. While the majority of horse ownership falls within the 35–54 age range, the final segment of horse owners extends into the 55+ group. This reflects the longevity of horse ownership as a lifelong activity and highlights the continued engagement of older adults in the equine industry.
