***********************************
Brakke Consulting’s
Animal Health News & Notes for September 8, 2023
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Vetoquinol
Other News
Affordable Pet Labs
Bactolife
Boehringer Ingelheim
Ceva
Felix Pharmaceuticals
Genus Lifesciences
Halo
IAMS
Merck
Novozymes
Nutreco
S2G Ventures
Vetpocket
ViAqua Therapeutics
ZyVet
*********************************************
Southwest Veterinary Symposium – SWVS 2023
San Antonio, Texas – Henry B. Gonzalez Convention Center
September 21-24, 2023
It’s Our 20th Anniversary Y’all! – Register TODAY! Southwest Veterinary Symposium — a partnership of the Arkansas, Louisiana, New Mexico, Oklahoma, and Texas Veterinary Medicine Associations which provides continuing education for veterinary professionals. Join us this September in San Antonio, Texas, where you can learn about the latest innovations and products while exploring our exhibit hall, network with other veterinary professionals, and choose from more than 400+ hours of quality CE/RACE-approved sessions.
**************************************
EARNINGS NEWS RELEASES
- Vetoquinol SA reported results for the first half of 2023. Sales were EUR 256 million ($274 million), down -5.4% on a reported basis and -4.6% at constant exchange rates. The Americas territory grew by +2.3% at constant exchange rates, driven by growth in the US market and the recent launch of Simplera. Group operating income recurring was EUR 39 million ($42 million), compared with EUR 44 million for the first 6 months of 2022. (company press release)
*********************************************
COMPANY NEWS RELEASES
- Merck Animal Health announced a voluntary recall of three batches of Banamine/Banamine-S (flunixin meglumine injection) 50 mg/mL in the US due to the presence of particulate matter observed during routine quality testing and reviews. (Pharmabiz)
- Boehringer Ingelheim, the Bill and Melinda Gates Foundation, the UK Government Foreign Commonwealth and Development Office, and GALVmed announce the creation of a new partnership to work on solutions to combat African animal trypanosomiasis (AAT), a disease of vertebrate animals affecting cattle, water buffalo, sheep, goats, horses, pigs, dogs, and other species. (company press release)
- Ceva Sante Animale received FDA approval for Firodyl (generic firocoxib) chewable tablets for use in dogs. (FDA)
- ZyVet Animal Health received FDA approval for generic Acepromazine Maleate Tablets for use in dogs. (FDA)
- Felix Pharmaceuticals received FDA approval for generic Firocoxib chewable tablets for use in dogs. (FDA)
- The FDA announced it has conditionally approved Genus Lifesciences‘s Fidoquel-CA1 (phenobarbital tablets) for the control of seizures associated with idiopathic epilepsy in dogs. (FDA)
- IAMS announced the launch of PETconnect by IAMS, a service that provides general nutrition and wellness advice and information from a licensed vet technician or a nutrition advisor in real time. (Pet Business)
- Novozymes and Bactolife announced a joint development and commercialization agreement to finish development and launch the biosolution Ablacto+, a functional feed additive which can potentially stabilize the gut of piglets and reduce the severity of post weaning diarrhea (PWD). (GlobeNewswire)
- Affordable Pet Labs announced the expansion of its in-home blood collection services, conducted by certified veterinary phlebotomists. Affordable Pet Labs is currently offering this specialized service in metropolitan areas in 12 states across the U.S. (einnews)
- Vetpocket, a digital resource app, announced the launch of Vetpocket 2.1, featuring calculators, reference cards, digital notebook, and more for veterinary professionals and students. (DVM360)
- Halo announced the launch of its newest dog collar, the Halo Collar 3, which contains advanced features in both the collar’s hardware and software design, creating an accurate GPS dog fence and real-time location on any cellular network worldwide. (Pet Business)
- ISRAEL ViAqua Therapeutics, a biotechnology company developing an orally administered RNA-particle platform to improve disease resistance in aquaculture, announced it has completed a $8.25M round led by S2G Ventures with participation from Rabo Ventures, The Trendlines Group Ltd., Agriline Limited, Nutreco, I-Lab Angels and Circle Investments LLC. (Biospace)
*********************************************
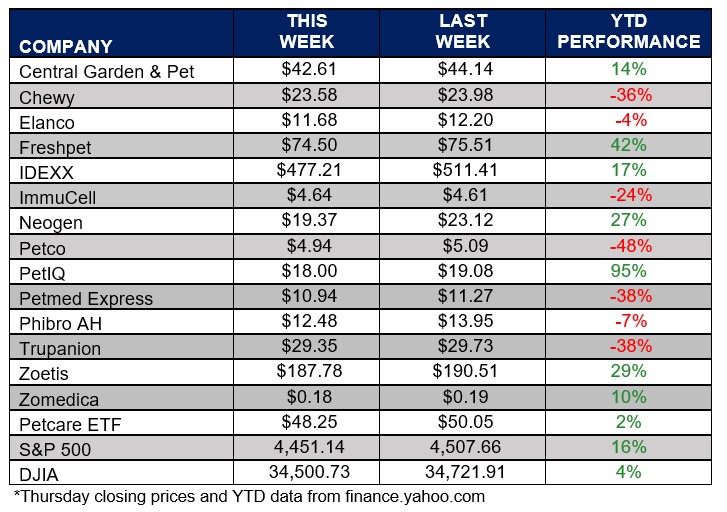
ANIMAL HEALTH STOCK PRICE TRACKER
***********************************
ANIMAL HEALTH NEWS
- SCOTLAND – VETERINARY SUPPORT Psychologists at the University of Aberdeen teamed up with veterinary professionals to develop a free toolkit help raise awareness of the adverse impacts associated with incivility, and support veterinary staff to mitigate and manage the impacts of uncivil behavior. The toolkit is designed to help veterinary staff to identify the types of incivility that may impact them negatively, provides a range of reflective tools, including scenarios, to help discussion and action around this issue and resources to help identify and implement measures to improve staff well-being. (ac.uk)
- US – ANIMAL ID The US Equestrian Federation’s Board of Directors has approved a new microchipping rule. Beginning December 1, 2025, under GR 1101.1, all horses competing in USEF-licensed or -endorsed competitions must be microchipped with a 15-digit ISO compliant 11784/11785 chip. US Equestrian has developed a microchip information webpage which contains outreach and educational resources. (association press release)
- US – VETERINARY TELEHEALTH For a third time a federal district court has upheld provisions of the Texas veterinary practice act requiring in-person establishment of a veterinarian-client-patient relationship (VCPR). The most recent ruling in the decade-long court battle has come after repeated challenges from a Texas veterinarian claiming the requirement violates his First Amendment right to free speech. (AVMA News)
- US – PET VACCINATION A recent study led by a researcher from the Boston University School of Public Health, highlights the concerning levels of canine vaccine hesitancy (CVH) in the US. The study reveals that over half (53%) of dog owners express some level of skepticism about vaccinating their pets, citing concerns about safety, efficacy, and necessity. The study found nearly 40% of dog owners believe canine vaccines are unsafe, more than 20% consider them ineffective, and 30% perceive them as medically unnecessary. Additionally, about 37% of dog owners believe canine vaccination could cause autism in their pets. The study identifies a “vaccine spillover” effect, indicating how individuals who hold negative attitudes toward human vaccines are more likely to exhibit the same skepticism toward pet vaccinations. This alignment of views extends to opposition to policies promoting widespread rabies vaccination in dogs. The research was published in the journal (Veterinary Practice News)
************************************
BRAKKE CONSULTING VIEWPOINT
Companion animal parasiticides represent the single largest market segment in the US Animal Health market. Estimates peg the prescription portion of this market segment at approximately $7.0 billion at the retail level. As a result of this market opportunity, many of the animal health companies look to innovate and introduce new products and technologies into this segment.
The latest innovation introduced into the US market are triple combination products providing convenient flea, tick and heartworm protection in a single monthly chew. Simparica Trio was the first to market and has been a huge success. On July 20, 2023, Boehringer announced the approval of Nexgard Plus. What’s next in this market segment?
On May 1, 2023, Merck Animal Health announced the approval of Bravecto Quantum in Australia, the first injectable flea and tick product, providing 12 months of coverage with a single dose to our furry family members. Convenience, compliance combined with efficacy appears to be the direction that the parasiticide market is headed. Zoetis confirmed in their May 2023 investor day presentation that they are also working on long-acting injectable products. The race is indeed on.
Will we see the transformation of this huge market from oral to injectable administration? What will this do to the oral or topical parasiticides retail or home delivery business? Have a crystal ball?
Will you switch from oral to injectable flea and tick products once the injectable parasiticides are approved for use in the US?
Randy Freides
***********************************
YOUR VIEW
Last week, we asked you what percentage of Chewy’s 2Q sales were from autoship orders. 31% said less than 50%, 20% said 55%, 30% said 65%, 17% said 75%, and 2% thought it was 85%. The answer: 76% – wow!
