***********************************
Brakke Consulting’s
Animal Health News & Notes for December 18, 2020
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Avivagen
Other News
APC Microbiome Ireland
California Pet Pharmacy
Companion Animal Health
DJO
Elanco (KindredBio)
Elanco (Tarsus)
Farmina Pet Food
ImmuCell
Impossible Foods
Kindred Biosciences
Linnaeus
LiteCure
Mars
Nestle Purina PetCare
Pets at Home
Resilient Biotics
Revivicor
Tarsus Pharmaceuticals
Vaxxinova US
Wagmo
White Dog Labs
Zoetis (Vaxxinova)
Zoetis (Solensia)
Zomedica
************************************
COMPANY EARNINGS RELEASES
- Avivagen reported its financial results for the fiscal year ended October 31, 2020. The Company reported revenues of C$1,177,857 ($977,451 in the prior fiscal year) and a comprehensive loss of C$(4,751,287) compared to a comprehensive loss of $(4,836,420) in the prior-year period. (company press release)
************************************
COMPANY NEWS RELEASES
- Elanco Animal Health announced an agreement with Kindred Biosciences to acquire exclusive global rights to KIND-030, a first-of-its-kind monoclonal antibody being developed for the treatment and prevention of canine parvovirus (CPV). The global license includes an upfront payment of $500,000 and additional milestone payments; sales performance will result in royalties and additional milestone payments for achieving revenue targets. Additionally, as part of the agreement, KindredBio also has a right of last refusal to manufacture certain other Elanco monoclonal antibodies. (company press release)
- Elanco announced it has expanded its agreement Tarsus Pharmaceuticals, which in 2019 was granted worldwide development and marketing rights to lotilaner (the active ingredient in Credelio) to develop treatments for any eye or skin diseases in humans. The new agreement gives Tarsus the development and marketing rights of lotilaner for all other applications in humans. (IHS Markit Connect)
- Vaxxinova US, formerly Epitopix, announced that marketing rights for the SRP Salmonella cattle vaccine, previously marketed by Zoetis, have reverted to its manufacturer Vaxxinova US. (Hoards Dairyman)
- Nestle Purina PetCare announced that, roughly one year after the grand opening of a $320 million wet pet food facility in Hartwell, Georgia, it will invest another $550 million to expand the facility and its workforce. New processing and packaging lines, as well as expanded warehouse capacity, will be added to the Hartwell facility to meet growing demand for Purina’s wet pet food brands. (GlobalPets)
- Zomedica announced that while a majority of shareholders voted in favor of the Company’s proposed domestication to Delaware, shareholders failed to approve the proposal by the necessary 2/3 vote and therefore the domestication will not be implemented. The company will remain domiciled in Canada. (company press release)
- Italy-based Farmina Pet Food announced it is spending $28.5 million to establish a new North American headquarters in North Carolina, including manufacturing, R&D and warehousing operations. (GlobalPets)
- LiteCure, the parent company of Companion Animal Health, announced it has been acquired by DJO LLC, a maker of braces, supports and orthopedic devices used in human medicine. The purchase of LiteCure puts DJO in the veterinary space for the first time. Terms of the transaction were not revealed. (Todays Veterinary Business)
- Wagmo, a tech-first pet insurance company, announced a partnership with California Pet Pharmacy (CPP) to provide nationwide access to flea, tick and heartworm medications for Wagmo customers. California Pet Pharmacy is licensed to dispense medications in all 50 states This benefit is available to customers as part of the Wagmo Perk Program. (PRnewswire)
- The FDA has approved Revivicor‘s first-of-its-kind intentional genomic alteration (IGA) in a line of domestic pigs, referred to as GalSafe pigs, which may be used for food or human therapeutics. This is the first IGA in an animal that the FDA has approved for both human food consumption and as a source for potential therapeutic uses. The IGA in GalSafe pigs is intended to eliminate alpha-gal sugar on the surface of the pigs’ cells, potentially providing a source of porcine-based materials to produce human medical products that are free of detectable alpha-gal sugar. People with Alpha-gal syndrome (AGS) may have mild to severe allergic reactions to alpha-gal sugar found in red meat (e.g., beef, pork, and lamb). Click here for more information. (FDA)
- At a recent Web Summit conference, the founder and chief executive of Impossible Foods reiterated the company’s plan to contribute to the end of the animal-based food sector, adding that the company’s mission is “to completely replace the use of animals as a food technology by 2035.” (IHS Markit Connect)
- Resilient Biotics announced it has secured $7.1 million in funding from undisclosed investors to develop microbiome-derived live biotherapeutics for veterinary applications. The company’s lead candidate is an antibiotic alternative for bovine respiratory disease (BRD). (IHS Markit Connect)
- White Dog Labs (WDL) announced an investment from a group of venture capitalists based in Oman to help WDL repurpose a recently purchased biorefinery in Minnesota for the production of high-quality animal-free protein. The company will use this protein to produce a line of dairy and meat substitute products for humans and animals. (IHS Markit Connect)
- EU Zoetis announced that the European Medicines Agency’s Committee for Medicinal Products for Veterinary Use (CVMP) adopted a positive opinion for Solensia (frunevetmab), an injectable solution for the alleviation of pain associated with osteoarthritis in cats. The felinized monoclonal antibody inhibits nerve growth factor-mediated cell signaling. The product, scheduled to be commercialized in 2021, will be the first monoclonal antibody medicine authorized for cats. (IHS Markit Connect)
- IRELAND ImmuCell and APC Microbiome Ireland announced a collaboration that will see ImmuCell benefit from APC’s intellectual property and capabilities relevant to animal health. APC’s portfolio includes patented microbiome-derived solutions for bovine mastitis and know-how of bioengineering antimicrobial peptides for increased efficacy. (Foodingredientsfirst.com)
- UK Mars’ Linnaeus division has agreed to buy five specialty referral centers in Britain from Pets at Home. Pets at Home will get an initial GBP 80 million ($106.6 million) payment from Linnaeus and up to GBP 20 million ($26.6 million) in performance payments contingent on future financial milestones being met by the referral centers. (Vet Advantage – VIN News)
***********************************
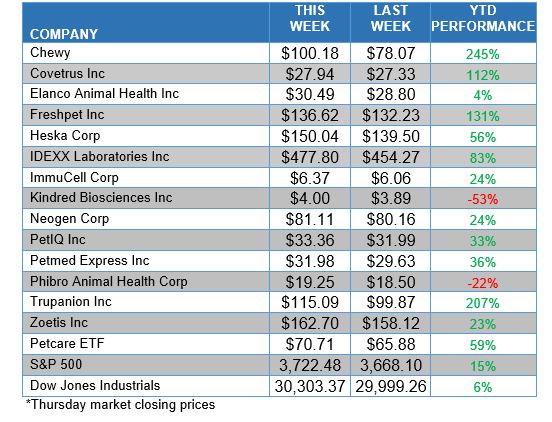
ANIMAL HEALTH STOCK PRICE TRACKER
 ***********************************
***********************************
ANIMAL HEALTH NEWS
- UK – VETERINARY SUPPORT Vets in Mind Alliance announced the launch of its free app to support the health and welfare of all members of the veterinary profession. The App is provided by a not-for-profit consortium of interested parties and funded by Petplan Charitable Trust, the North American Veterinary Community, Circa Healthcare and Vetstream; as well as members who are providing content and marketing services. The App is available from the Apple App Store and Google Play Store. Click here for more information. (association press release)
- US – EXPORT The FDA announced the launch of a new online portal to assist industry with exporting animal products. Effective January 1, 2021, the FDA’s Center for Veterinary Medicine (CVM) will no longer issue certain certificates on unique CVM paper with a gold seal; exporters will now be able to access these certificates online and print them on their own. Click here for more information. (FDA)
- US – SARS-COV2 A wild mink found and tested near an infected fur farm in Utah is the first free-ranging native animal known to have tested positive for SARS-COV-2, the virus that causes COVID-19. In October thousands of minks at fur farms in Utah reportedly died from the disease, forcing nine sites in three counties to quarantine. (Salt Lake Tribune)
- US – ANTIBIOTICS The FDA’s Center for Veterinary Medicine published the 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Click here for more information. (FDA)
- US – FMD VACCINE USDA’s Animal and Plant Health Inspection Service is reopening a comment period to evaluate a petition from Zoetis to manufacture foot-and-mouth disease vaccine in the United States. Public comments on Zoetis’ proposal can be submitted online here to APHIS through January 4. (Vet Advantage)
************************************
BRAKKE CONSULTING VIEWPOINT
This week CEO Pat Brown of Impossible Foods was quoted saying “Our mission is to completely replace animals as a food technology by 2035.” and “It’s game over for the incumbent industry – they just don’t realize it yet.” This message is consistent with other interviews that I have heard with Dr. Brown in the past. Along with the Impossible Burger, his company has now launched Impossible Sausage and Impossible Pork in 2020. Although Impossible Foods is not yet publicly traded, it is reported that the company has raised over $1.5 billion since its inception in 2011 and is estimated by Fortune magazine to be worth at least $4.0 billion today, with annual sales estimated at $151.5 million. It’s IPO is eagerly awaited by the market, and in the past 2 months the company has begun to sell product outside the US for the first time by launching its products in retail outlets in Hong Kong and Singapore.
Compare this information with the results of our survey last week. We asked you, “How do you think cultured protein will impact the traditional protein market?” 17% of respondents said it would take market share from animal protein in a flat market, 36% think that it will bring new customers and expand the market, 22% think it will have no impact, and 25% think that demand for animal protein will decrease and market share will move to cultured protein.
So, the question for the 75% who think that cultured or plant-based “meat” will coexist with our traditional animal protein sources, your largest competitor in the cultured protein space has the stated goal of putting you out of business. It is growing sales and raising money at an astronomical rate, and it is surrounded by other companies chasing similar technologies (although getting less public attention). What will you do to stay relevant and healthy between now and 2035?
Jim Kroman
***********************************
YOUR VIEW
This week let’s get your view on your Christmas holiday celebration plans and we will let you know the results in two weeks.
This week
Given the current situation of COVID-19, will you be spending time at Christmas with someone outside your “pod,” that is, someone who does not normally live in your household? If yes, will you travel, and how?
