***********************************
Brakke Consulting’s
Animal Health News & Notes for May 29, 2020
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Alivira
Animalcare
Kane Biotech
Krka
Pets at Home
Other News
Anivive Lifesciences
Ascus Biosciences
Bayer
Boehringer Ingelheim
Cargill
Eagle Genomics
Eden Research
Hill’s Pet Nutrition
Petmedix
Royal Canin
Vetoquinol
Victor Medical
ViroVet
VitusVet
Zomedica
************************************
REGULATORY WORKSHOP
Animal Health Regulatory 101: An Introductory Course on the Regulatory Aspects of Animal Drug and Vaccine Development
PRESENTED BY KANSAS STATE UNIVERSITY OLATHE
August 26 & 27, 2020 (on-site and remote attendance options available)
Kansas State University Olathe is hosting a two-day workshop on August 26 & 27, 2020 that will provide attendees with the regulatory acumen necessary to make smarter business decisions in management and leadership in the animal health industry. This program provides an overview of essential regulatory guidelines, terminology, concepts and applications in the day to day animal health business.
Registration for this information-packed two-day workshop is $1,500 per person. For more information or to register, click here.
************************************
COMPANY EARNINGS RELEASES
- SeQuent Scientific Limited announced its financial results for the fiscal year ended March 31, 2020. Animal Health (Alivira) revenues were $166.4 million, an increase of 13.5% over the prior year. EBITDA was $24.8 million, an increase of 32.5%. (company announcement)
- Krka reported results for the first quarter of 2020. The Slovenian firm posted animal health revenues of EUR 22.2 million ($24.3 million), an increase of 26% compared to the prior-year quarter. (Animal Pharm)
- Animalcare reported results for the full year 2019. Revenues for fiscal 2019 came to GBP 71.1 million ($87.3 million), a 2% decline compared to the prior year. Animalcare’s companion animal segment grew sales by 1% to GBP 46.4 million ($57 million) in 2019. (Animal Pharm)
- Kane Biotech announced its first quarter 2020 financial results. Total revenues were $456,139, a decrease of 27% compared to the same period in 2019. Loss for the first quarter of 2020 was $(1,363,836) compared to a loss of ($657,393) for the quarter ended March 31, 2019. (company press release)
- Pets at Home reported preliminary results for its fiscal year 2020. Total Group revenue was GBP 1,058 million ($1,289 million), an increase of 10% over the prior year. (Globalpets.com)
************************************
COMPANY NEWS RELEASES
- Eden Research PLC announced that its license agreement with Bayer Animal Health has been amended as there will be increased investment in the project, in part financed by Eden’s successful March fundraise. The companies are working on an animal shampoo, a conditioner, a spray, and an ear flush using Eden’s encapsulation technology. (Proactiveinvestors.co.uk)
- The FDA announced it has approved Vetoquinol USA’s Imoxi Topical Solution for Cats, the first generic version of a topical imidacloprid and moxidectin product for cats. (FDA)
- Hill’s Pet Nutrition announced it has partnered with The Association for Animal Welfare Advancement to provide $400,000 in grants to animal shelters impacted by COVID-19. Applications for Hill’s Disaster Relief Grants will be accepted until June 2 and will be paid out in full by Aug. 31. Eligible applicants can visit Hill’s Support for Shelters to learn more and/or apply. (Pet Business)
- Royal Canin announced that it has expanded its line of gastrointestinal diets with the first and only formulas designed specifically for kittens and puppies. (Veterinary Practice News)
- Zomedica Pharmaceuticals announced the pricing of a public offering of 133,333,333 common shares (or common share equivalents) of the Company, together with warrants to purchase up to 133,333,333 common shares, at a combined public offering price of $0.15 per share and accompanying warrant. The gross proceeds from this offering are expected to be approximately $20.0 million, before deducting placement agent’s fees and other estimated offering expenses. (Globenewswire)
- Anivive Lifesciences has submitted an application to the US FDA to repurpose its GC376 feline drug for COVID-19 in humans. Since 2018, Anivive has been developing its GC376 candidate to treat feline infectious peritonitis (FIP), which is caused by a coronavirus. GC376 was identified as a potential COVID-19 treatment by AniviveSELECT, which is the firm’s artificial intelligence-powered drug discovery software. (Animal Pharm)
- VitusVet announced that it has been named a Preferred Client Communication Technology Partner for Victor Medical Company. The VitusVet platform will now be available at preferred pricing for all of Victor Medical’s 4,000 member practices. (Globe Newswire)
- Cargill and Eagle Genomics, a life sciences and microbiome knowledge discovery company, announced that they have signed a multiyear platform agreement to enable the digital transformation of microbiome and life sciences research and development (R&D) across Cargill’s global locations. (Feedstuffs)
- Ascus Biosciences announced the closing of a $46 Million series B funding round led by global investment company Temasek. Ascus has multiple microbial solutions in development, with some already in sales mode, for dairy, poultry, beef feedlot, equine, and companion species. (oaoa.com)
- EU Boehringer Ingelheim Animal Health announced that the European Medicines Agency’s Committee for Medicinal Products for Veterinary Use (CVMP) awarded positive opinion rulings to the company’s Prevexxion RN+HVT+IBD and Prevexxion RN poultry vaccines. Prevexxion RN+HVT+IBD provides active immunity in one-day-old chicks in order to prevent mortality and clinical signs, and reduce lesions caused by Marek’s disease virus and infectious bursal disease (also known as Gumboro disease) virus. Prevexxion RN contains live recombinant Marek’s disease virus (serotype 1, strain RN1250). Full approval of the two vaccines is expected in July. (Animal Pharm)
- BELGIUM ViroVet announced it has closed a series B funding of EUR 6 million ($6.6 million) to help the firm further develop and launch the products currently in its pipeline. ViroVet claims its platform allows it to design and produce thermostable vaccines at a faster and more cost-effective rate. (Animal Pharm)
- UK Petmedix announced that it has raised an equity investment from Cambridge Innovation Capital that will be used to advance its antibody technology platform to bring innovative medicines to companion pets. (company press release)
***********************************
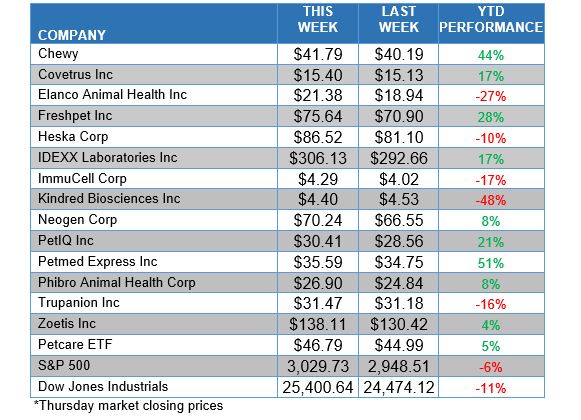
ANIMAL HEALTH STOCK PRICE TRACKER
 ***********************************
***********************************
ANIMAL HEALTH NEWS
- IRELAND – BSE Atypical bovine spongiform encephalopathy (BSE) has been confirmed in a 14 year old Limousin cow in Ireland. The suspect animal was sampled by DAFM staff as part of the ongoing official sampling of all fallen animals of 48 months and older. The OIE said all susceptible animals were culled and tested negative for BSE, and the case has now been resolved. (Beefcentral.com)
- US – HOG DISPOSAL The Iowa Department of Agriculture & Land Stewardship is launching a disposal assistance program to help pork producers who are unable to harvest pigs due to COVID-19 supply chain The department is offering producers $40 per approved animal to help cover some of the disposal costs for market-ready hogs (weighing at least 225 lb.). Iowa State University estimates that, as of mid-May, approximately 600,000 pigs in Iowa were unable to be harvested, despite pork producers donating pork to food banks and attempting to identify other markets for their animals. (Feedstuffs)
- UK – ASF RESEARCH Scientists from The Pirbright Institute in the UK have developed a vectored vaccine for African swine fever (ASF) that uses a non-harmful virus (the vector) to deliver eight strategically selected genes from the ASF virus genome into pig cells. In a recent trial, 100% of pigs immunized with the new vaccine survived a lethal dose of ASF virus. The vaccine also will enable the differentiation of infected animals from those that have received a vaccine. The research was published in (Animal Pharm)
- UK – CATTLE GENETICS The Roslin Institute announced it has created a library of cattle genes to help understanding of inherited traits linked to animal health. The latest reference work builds on a gene expression atlas that was created around a decade ago, which was based on the genetic code of a single cow. The atlas can be found here. (Animal Pharm)
- US – PET INSURANCE The North American Pet Health Insurance Association (NAPHIA) released its State of the Industry 2020 report. The 2019 results, included in NAPHIA’s 2020 State of the Industry Report, reported that North America’s pet health insurance sector reached $1.717 billion (USD) in 2019, a 21% improvement over its 2018 performance. Over 2.81 million pets were insured across North America in 2019. (association press release)
************************************
BRAKKE CONSULTING VIEWPOINT
Having worked in animal health companies that were a little part of big human pharmaceutical companies, I’ve always hoped that the little guys could teach the big guys something, someday, when it comes to science. We hear how difficult it is to bring a human vaccine to market and, combined with the fact that there is no vaccine for SARS or MERS, maybe it’s unlikely we will ever have a vaccine against the SARS-CoV-2 virus.
But today we have coronavirus vaccines used in cattle, swine, poultry, dogs and cats, which have recently been reviewed here. So, it can be done and maybe the veterinary industry will indeed help with human vaccines and antivirals.
The coronavirus that causes Feline Infectious Peritonitis (FIP) in cats is probably the most difficult to prevent by vaccination, and treatment is problematic. A young company, Anivive, might just have an answer to this serious, fatal disease in cats. Their lead drug candidate, GC376, a selective protease inhibitor, has been shown to be safe and shows rapid improvements in clinical signs in cats in 24-36 hours. Now the company has filed a pre-IND with the FDA for treatment of COVID-19 in humans. It seems to work in vitro. Maybe a little guy will teach the big guys something someday soon. We hope they do.
Bob Jones
***********************************
YOUR VIEW
Last week we asked about the frequency of wearing a mask and we received many responses – thanks! A little over two thirds of you said that you routinely wear a mask when you go out in public and over half of you said that a majority of people in your area are wearing masks when out in public. It looks like our readers are, as expected, a little above the average!
For this week’s question, we’re sticking with the pandemic theme again. Quite a few conventions and conferences have been cancelled stretching into the summer and fall. Knowing what you know now, how would you answer this question today?
When will you be ready to attend one of your usual company or industry conferences or conventions?
