***********************************
Brakke Consulting’s
Animal Health News & Notes for July 17, 2020
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Other News
Antech Diagnostics
Bayer
Biomin
Elanco (Bayer)
Elanco (Neogen)
Dechra
Gravity Payments
GrowSafe Systems
HoverHeat
Invetx
Kemin Industries
LexaGene
Neogen (Elanco)
Neogen (Soleris)
PetIQ
Twist Bioscience
Vet2Pet
VetCell Therapeutics
Vytelle
Wheatsheaf Group
Zoetis
************************************
COMPANY NEWS RELEASES
- Elanco Animal Health Incorporated announced that the company has received unanimous approval from the U.S. Federal Trade Commission (FTC) for its acquisition of Bayer Animal Health. The FTC’s approval is conditional on the following proposed divestitures: Worldwide rights for Elanco’s Osurnia, a treatment for otitis externa in dogs, being sold to Dechra Pharmaceuticals PLC; US rights for Elanco’s Capstar, an oral tablet that kills fleas in dogs and cats, being sold to PetIQ, Inc.; US rights for Elanco’s StandGuard, a pour-on treatment for horn fly and lice control in beef cattle, being sold to Neogen Corporation. The FTC decision represents the final antitrust clearance needed to complete the transaction, which continues on track for closing at the beginning of August. Elanco also received consent from Canada’s Competition Bureau, pending the divestiture of Osurnia and Bayer’s Profender, as well as Elanco’s commitment to forego acquiring the Canadian distribution rights to Bayer’s Tempo, Credo, QuickBayt and Annihilator Polyzone poultry insecticides. (company press release, Newswire.ca)
- The USDA’s Animal & Plant Health Inspection Service (APHIS) is seeking public comment on a petition from Zoetis seeking approval to produce foot and mouth disease (FMD) vaccine consisting of a modified non-infectious and non-transmissible strain of the virus on the US mainland. The manufacturer asserts that because the FMD virus is no longer able to produce infection, it should not be considered a live virus of FMD and should be able to be produced on the US mainland. Public comments will be accepted through Sept. 14, 2020. To submit a comment, click here. (USDA, Feed Strategy)
- Neogen Corporation announced that it plans to acquire the US (including territories) rights to Elanco’s StandGuard Pour-on for horn fly and lice control in beef cattle, and related assets. Financial terms were not disclosed. (company press release)
- Antech Diagnostics announced the launch of the Canine CE-IBD assay, designed to determine whether a dog’s chronic gastrointestinal symptoms correspond with Inflammatory Bowel Disease (IBD). The test relies on three novel serologic biomarkers to diagnose and manage IBD. (IHS Markit Connect)
- Neogen Corporation announced the launch of Soleris Next Generation (NG), an advanced test system that automatically detects microorganisms in a fraction of the time of traditional testing methods. (company press release)
- Kemin Industries announced that it has acquired a US patent application from the Kansas State University Research Foundation for a method to control African swine fever virus (ASFV) in feed and feed ingredients using its Sal CURB Liquid Antimicrobial pathogen control product. (Feedstuffs)
- VetCell Therapeutics announced it has received authorization from the FDA to start a country-wide investigational clinical trial, in collaboration with researchers from the UC Davis’ Veterinary Institute for Regenerative Cures, of its DentaHeal cell therapy product for treatment for feline chronic gingivostomatitis (FCGS). DentaHeal is an allogenic, adipose-derived mesenchymal stem cell (MSC) therapy. (IHS Markit Connect)
- Vytelle and GrowSafe Systems, Ltd. announced the combining of their companies with the aim of accelerating genetic advances in bovine biotechnology. The companies will operate as a single precision livestock company under the Vytelle brand. With the combined capabilities of the new Vytelle, cattle producers will be able to convert individual animal performance data into genetic progress. Both companies are owned by UK-based Wheatsheaf Group. (company press release)
- Vet2Pet and Gravity Payments announced they have partnered to bring virtual payments to veterinary hospitals in the US. (company press release)
- The inventor of the Hug-U-Vac surgical positioning system for human and veterinary patients has released another product: the HoverHeat warming device. HoverHeat supports normal body temperature when placed under a veterinary patient and connected to a warm air blower. (Todays Veterinary Business)
- Invetx announced that it has partnered with Twist Bioscience to engineer and optimize monoclonal antibodies as part of its novel discovery platform to create therapeutics for veterinary animals, joining existing collaborators AbCellera and Wuxi Biologics. (company press release)
- LexaGene announced it expects to launch its MiQLab, a fully-automated genetic analyzer, in September. The MiQLab is designed to deliver reference laboratory-quality data at the point of care. The technology can screen samples for up to 27 different targets simultaneously to detect pathogens and antimicrobial resistance factors, and can generate results in around one hour. (IHS Markit Connect)
- EU Biomin announced it has secured European zootechnical authorization for the use of new capsule technology in its Digestarom DC Power feed additive for poultry. The capsule technology ensures plant-based active compounds in the feed additive are protected during feed processing and are released through the gastrointestinal tract slowly for maximum effectiveness. (IHS Markit Connect)
***********************************
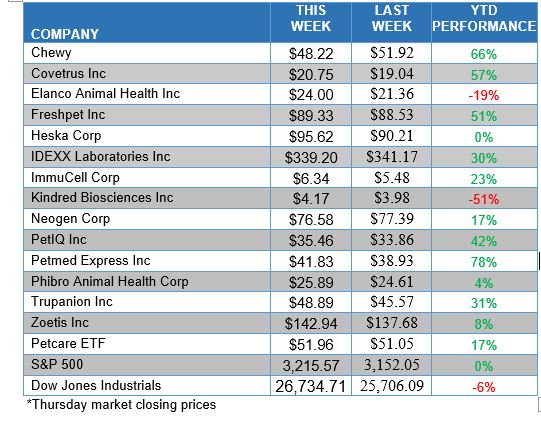
ANIMAL HEALTH STOCK PRICE TRACKER
***********************************
ANIMAL HEALTH NEWS
- US – CWD The USDA’s Animal & Plant Health Inspection Service (APHIS) announced that it is making available up to $3.5 million for state and tribal governments to carry out research and management activities to help combat chronic wasting disease (CWD) in farmed and wild deer and elk populations. Priority will be given to areas that have already detected CWD and implemented CWD monitoring and surveillance programs or that propose to create control programs. (Feedstuffs)
- US – MUMS The FDA has released revised draft guidance to help researchers and animal drug sponsors navigate the pathway to approval for animal drugs intended for minor uses and minor species (MUMS). Draft Guidance for Industry #61, entitled “Special Considerations, Incentives, and Programs to Support the Approval of New Animal Drugs for Minor Uses and for Minor Species,” will be available for comment for 120 days, starting July 15, 2020 through November 12, 2020. Click here for more information. (FDA)
- US – FDA The FDA announced the availability of four draft guidance documents to help facilitate the development of New Animal Drug submissions. Click here for more information. (FDA)
************************************
BRAKKE CONSULTING VIEWPOINT
In some respects, the underlying theme of this week’s newsletter is opportunity. On the one hand, when companies merge, especially large companies, there are opportunities for other firms, especially smaller ones, to pick up new products that were spun off. On the other hand, there are several news items about new products and technologies that can open up new markets in animal health. Many are developments from companies that you may not have heard of before. All in all, it demonstrates the vibrancy of our industry, an important characteristic at a time when there are major challenges. Be glad that you picked animal health and nutrition for a career instead of, say, hospitality and travel.
John Volk
***********************************
YOUR VIEW
Last week we ask your expectations for 3Q20. 41% think results will be better than expected. 53% said worse. 6% hadn’t heard back from their tarot card reader yet.
This week’s poll
This week we want to know about veterinary conferences. As you know, most conferences are being hosted online rather than in person.
If you normally go to conferences in person, are you likely to attend those same conferences virtually this year? [yes or no]
Likewise, if your company typically exhibits at veterinary conferences, will you exhibit at the online versions this year or sit this one out? [Will exhibit. Will not exhibit. Haven’t decided yet.]
Click here to share your thoughts.
