***********************************
Brakke Consulting’s
Animal Health News & Notes for January 8, 2021
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Earnings News
Neogen
Other News
Adler Laboratories
ADP
Alergovet
Artuvet
BarkBox
Bimeda
Boehringer Ingelheim
BrightPet Nutrition Group
Bullymake
Creso Pharma Limited
Dr. Baddaky
DRN
Holden2
ICF
Manna Pro Products
Megazyme
Midwestern Pet Foods
MiracleCorp
Neogen (Reveal)
Neogen (Megazyme)
Nextmune
Northern Star Acquisition
Pet Supplies Plus
Pet Valu
PetVivo
Phibro Animal Health
PromoVet
Saiba Animal Health
Spectrum Vet
Swedencare
University Products
VetPartners
VetriScience Labs
Vetruus
Virbac
Zomedica
************************************
COMPANY EARNINGS RELEASES
- Neogen Corporation announced results for the second quarter of its 2021 fiscal year, which ended Nov. 30, 2020. Revenues were $115 million, a 7% increase compared to the previous year. Second quarter net income was $15.9 million compared to the prior year’s $16.3 million. The Animal Safety segment reported revenues of $57.5 million, an increase of 13% compared to the prior-year quarter. (company website)
************************************
COMPANY NEWS RELEASES
- Boehringer Ingelheim Animal Health announced an agreement with is working with Swiss firm Saiba Animal Health to develop vaccines to treat chronic inflammatory diseases, cancer and allergies in companion animals from a platform based on non-infectious virus-like particles (VLPs). Saiba claims it can easily adapt its VLP vaccine platform to target any disease-associated molecule, specifically monoclonal antibody therapies. (IHS Markit Connect)
- The FDA announced the approval of Bimeda’s SelaSpot (generic selamectin topical) for use in dogs and cats. (FDA)
- VetriScience Labs announced the launch of VetriFLEX Feline Formula supplement specifically designed for cats to provide superior joint support. (company announcement)
- Zomedica Corp. announced the appointment of Robert Cohen as Chief Executive Officer effective January 1, 2021. Mr. Cohen previously served as the Company’s Interim CEO and as a member of the Board of Directors. (company press release)
- Neogen announced the launch of Reveal tests to determine meat speciation for horse, beef, sheep and poultry in raw meat and environmental samples in just 5 minutes after a simple water extraction. The new tests can detect as little as 0.5% of the target species in raw meat or raw processed samples and have also been validated for use with environmental swabs and rinse water samples. The new tests join Neogen’s existing Reveal for Pork in the company’s simple and quick meat speciation test range. (Feedstuffs)
- Nextmune announced that the company’s businesses – Baddaky (Scandinavia), Vetruus (UK), Artuvet (Germany, Benelux), Alergovet (Iberia) and Spectrum Vet (US) – will begin operating under a new name and will be known as Nextmune starting January 1, 2021. The operations in Italy will remain ICF and DRN and typically be referred to “ICF, a Nextmune company” and “DRN, a Nextmune company,” respectively. The company’s product brands will remain unchanged. (Biospace.com)
- University Products LLC officially announced the creation of a vaccine that has been successfully used to treat bovine anaplasmosis since 2000. Developed at the Agriculture Center of Veterinary Science at Louisiana State University and approved by the USDA for experimental use, the vaccine has already been widely deployed with no side effects or adverse reactions reported. So far, more than two dozen states and Puerto Rico have approved its use. (Feedstuffs)
- BrightPet Nutrition Group announced it has acquired MiracleCorp and its portfolio of assets. MiracleCorp sells freeze-dried and other pet treats under the Stewart brand. Financial terms were not disclosed. (Pet Business)
- Manna Pro Products announced it has acquired Bullymake, a direct-to-consumer dog subscription box built specifically for power chewers. Financial terms were not disclosed. (Pet Product News)
- Swedencare announced it has acquired US e-commerce specialist Holden2 for $21 million. Holden2 sells products under the PetMD brand through online platforms such as Amazon and Chewy. Holden2 grew by almost 100% from October 2019 to September 2020, recording annual sales of $15.8 million (with adjusted EBITDA of $2.5 million). (IHS Markit Connect)
- PetVivo, maker of Kush toys, announced a 1-for-4 reverse stock split of its shares to improve its capital structure ahead of a planned Nasdaq listing. The shares began trading on a split-adjusted basis on December 30. (IHS Markit Connect)
- Pet Supplies Plus announced it will acquire an estimated 40 previously operated Pet Valu locations throughout Indiana, Kentucky, Maryland, New Jersey, Ohio, Pennsylvania and Virginia with some locations to reopen with Pet Supplies Plus branding, products and services as early as January, 2021. The former Pet Valu stores will be a mix of corporate and franchisee-owned Pet Supplies Plus locations. Financial terms were not disclosed. (Pet Age)
- BarkBox and Northern Star Acquisition have entered into a definitive merger agreement, officials reported in mid-December. As a result of the transaction, which officials value at about $1.6 billion, BarkBox will become a publicly listed company of the New York Stock Exchange under the new ticker symbol “BARK.” (Pet Product News)
- The FDA announced that certain Sportmix pet food products manufactured by Midwestern Pet Foods, Inc. may contain potentially fatal levels of aflatoxin. The FDA is aware of at least 28 deaths and 8 illnesses in dogs that ate the recalled product. For more information and a list of the affected products, click here. (FDA)
- PromoVet announced it has partnered with ADP to offer payroll and human resources management services to PromoVet members. (company press release)
- CANADA Virbac and Phibro Animal Health announced an agreement under which Phibro will represent and market Virbac’s newly approved TULISSIN 100 brand of tulathromycin, which is indicated for use in cattle, swine and sheep. (GlobalBankingandFinance.com)
- IRELAND Neogen announced the acquisition of Ireland-based Megazyme Ltd., a supplier of analytical products deployed in food and beverage quality control laboratories. Megazyme will be run as a standalone company managed through Neogen’s European base in Scotland. Financial terms were not disclosed. (mibiz.com)
- LATIN AMERICA Creso Pharma Limited announced it has secured regulatory approval from the Ministry of Agriculture and Animal Feed in Uruguay through its commercial partner Adler Laboratories, Uruguay, for its line of animal health products, anibidiol. According to the company, the approval effectively means CPH’s anibidiol becomes the first approved CBD hemp-based complementary feed for pets in Latin America. (Finfeed.com)
- ITALY UK-based VetPartners announced it has acquired four Italian veterinary practices as part of its ongoing European expansion. Financial terms were not disclosed. (bdaily.co.uk)
***********************************
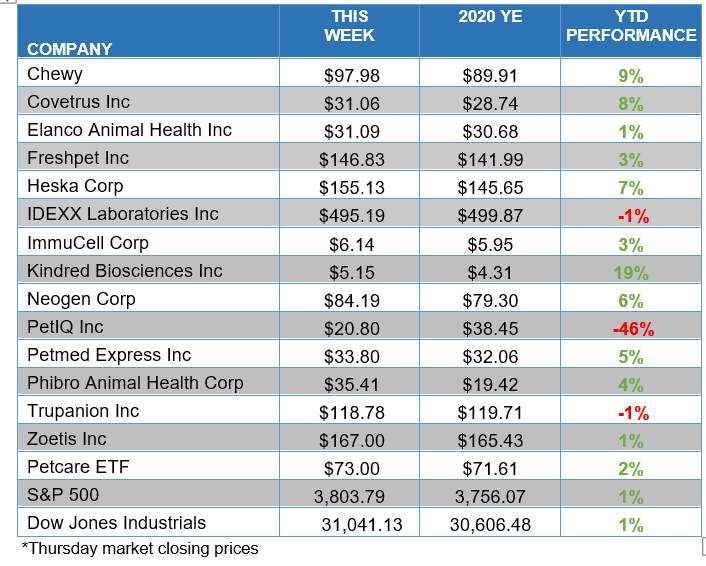
ANIMAL HEALTH STOCK PRICE TRACKER
***********************************
ANIMAL HEALTH NEWS
- US – CORONAVIRUS The Oregon Department of Agriculture announced that a wild mink received a positive test from the USDA National Veterinary Services Laboratory, confirmed on December 22 after it was captured on December 13. The mink reportedly escaped from a mink farm in Oregon that has been under quarantine since ten mink samples tested positive for the coronavirus near the end of November. (Dailymail.co.uk)
- US – GMO REGULATION The U.S. Secretary of Agriculture has proposed transitioning oversight of bioengineered food animals from the FDA to the USDA. Under the proposed plan, USDA would become the agency responsible for determining whether genetically engineered animals are safe to eat. The agency would oversee reviews of the safety and efficacy of genetic modification in the animals, their impact on the environment and overall food safety for the bioengineered products. USDA would consult with the FDA on regulation and the FDA would still regulate genetically modified seafood and animals that have been bioengineered for medical reasons. (Vet Advantage – WattAgnet)
- US – GLOBAL PET EXPO The American Pet Products Association (APPA) and the American Pet Products Association (APPA) announced that amid continued safety concerns resulting from the global pandemic, Global Pet Expo will be moving to a fully-digital experience. Global Pet Expo Digital Access will take place March 24–26, 2021. Details for the event are being finalized. (Globalpets.com)
- US – VETERINARY TRAINING The American Veterinary Medical Foundation board of directors voted at its November 2020 meeting to provide $80,000 in funding to create an AVMA certificate program for veterinary first responders. The AVMA Committee on Disaster and Emergency Issues will first identify core competencies reflecting the basics that every veterinary responder should know. After that, organizations can develop new or modify existing courses and submit them to the CDEI for an assessment as to whether they satisfy one or more of the core competencies required for certificate completion. Once veterinarians or veterinary students complete courses that meet all core competencies, they will be issued the Basic Veterinary Responder Certificate. (AVMA)
************************************
BRAKKE CONSULTING VIEWPOINT
Judging by the first week of 2021, this is going to be a very busy year in the animal health industry. Already we see acquisitions, partnerships and new drug and vaccine product launches making loud headlines. A quieter headline dealing with GMO regulation in animals might lead to seriously big headlines in time.
The news of potential regulatory clarity between the FDA and the USDA regarding the food safety of bioengineered food animals is good for innovation and good for food animal production. There are two FDA approvals for genetically engineered animals, “AquaAdvantage” salmon (AquaBounty) and the “GalSafe” pig (Revivicor), but food products from these animals are not currently in the food chain. The long regulatory experiences based on these two approvals likely shaped the new proposal.
Maybe 2021 will be the year that we will more clearly see the benefits of using the CRISPR/Cas9 gene editing technology in food animals. Disease and parasite resistance, beneficial production traits, and heat tolerance are some of the traits for which the animal food production industry would benefit from modified genetics. Consumer acceptance will be a hurdle, but soon we should see more headlines from companies that can now see the regulatory hurdle to genetic innovation a bit more clearly.
Bob Jones
***********************************
YOUR VIEW
The last polling question of 2020 dealt with how you were going to spend your Christmas with your “pod”. Almost half of the respondents said they were staying home with only family present, a quarter said they will stay home but will have visitors (family or friends) and a quarter said they would travel to see family and friends by car. We certainly hope everyone had a safe Christmas.
This week
Let’s see what you think about pork. The “GalSafe” pork mentioned above comes from a pig that has a genetic alteration that stops the production of alpha-gal, a specific sugar found in the meat. People that have alpha-gal syndrome (AGS), an increasingly common allergy to meat and animal products caused by ticks, are not able consume products that contain this sugar without the risk of a severe allergic reaction.
How likely would you be to consume “GalSafe” pork, whether or not you had AGS?
