***********************************
Brakke Consulting’s
Animal Health News & Notes for September 27, 2019
Copyright © Brakke Consulting
Editor: Lynn Fondon DVM MBA
************************************
IN THE NEWS:
Brakke Consulting news
Market research
Earnings News
Animalcare
General Mills
Neogen
Other News
Antech Diagnostics
Boehringer Ingelheim
Chr. Hansen
Huvepharma
Innovet Pet
International Genetic Solutions
Laboratorios Hipra
Neogen
Novogen
Roslin Technologies
Tindog
Zoetis
***********************************
BRAKKE CONSULTING, INC.
MARKET RESEARCH SERVICES
Look Before You Leap
Depend on Brakke Consulting to meet your company’s market research needs, whether it is:
- Assessing the feasibility of a new product or technology
- Identifying the opinions and trends of veterinarians, pet owners or producers
- Determining the current market for products
With our in-house database of veterinarians and the ability to custom-tailor surveys of a wide variety of target audiences, we provide reliable insights into this unique industry. We also produce multiclient reports in a number of high-interest animal health market areas. For more information, see our website or call us at 336-396-3916.
***********************************
COMPANY EARNINGS RELEASES
- Neogen Corporation reported financial results for the first quarter of its 2020 fiscal year ended Aug. 31. Revenues were $101 million, a 2% increase compared to the previous year’s first quarter. Net income was $14.7 million compared to $15.2 million in the previous year’s first quarter. Neogen’s Animal Safety segment reported revenues of $50 million a 6% increase compared to the prior year first quarter. Revenues from Neogen’s worldwide animal genomics business increased 17% in the first quarter of fiscal 2020 compared to the prior year. (company press release)
- Animalcare reported results for the first six months of 2019. Sales were GBP 36.1 million ($45 million), representing growth of 0.2% compared to the prior-year period (0.9% at constant exchange rates). (Animal Pharm)
- General Mills reported results for the first quarter of fiscal year 2020. Revenues for the pet food segment, including Blue Buffalo, were $368 million, representing organic growth of 7% compared to last year. Operating profits were $81 million, an increase of 458% compared to last year. (Petfood Industry)
***********************************
COMPANY NEWS RELEASES
- Neogen announced a collaboration with International Genetic Solutions (IGS) to enhance both company’s capabilities in beef cattle genetics. The collaboration is focused on improving “genomic impact” in the IGS platform, which provides science-based genetic predictions. The partnership will also enhance the research and development necessary to continue to improve Neogen’s Igenity Beef Profile. (Animal Pharm)
- Huvepharma announced it has received FDA approval for combination use of Monovet 90 (generic monensin) with other drug feed additive products in the manufacture of Type B and C medicated feeds. (company press release)
- Hansen announced the launch of Bovamine Dairy Plus, which includes four species of beneficial bacteria: unique strains of Lactobacillus animalis, Propionibacterium freudenreichii, Bacillus subtilis, and Bacillus licheniformis. (company press release)
- Antech Diagnostics announced the launch of the RenalTech test across the VCA North American network of veterinary clinics and hospitals. According to Antech, the test is the veterinary industry’s first artificial intelligence-driven predictive diagnostic tool for companion animals, allowing users to predict chronic kidney disease in a cat around two years before signs of the disease are actually visible. (Animal Pharm)
- Innovet Pet announced that they have acquired co, an app for helping dog owners expand their social circle and find new friends. Innovet Pet will change Tindog’s focus, from social networking to a website that focuses on providing online access to health services, such as living with a psychological disability. (GlobalPets)
- EU Zoetis announced that the European Commission has granted marketing authorization for Simparica Trio (sarolaner/moxidectin/pyrantel) chewable tablets, a once-monthly triple combination antiparasitic medication for dogs with, or at risk from, mixed external and internal parasitic infestations. Zoetis expects to launch Simparica Trio in the EU in the first quarter of 2020. (company press release)
- FRANCE Boehringer Ingelheim announced it has broken ground in Lyon, France on what it claims will be one of the largest biotechnology production sites for veterinary vaccines in Europe. Boehringer is investing EUR 200 million ($220.6 million) in construction of the site, which it said will significantly increase production capacities for antigens and vaccines against highly contagious diseases including foot-and-mouth disease (FMD) and bluetongue disease. (Animal Pharm)
- EU Laboratorios Hipra announced that the European Medicines Agency’s Committee for Medicinal Products for Veterinary Use (CVMP) has adopted a positive opinion for the company’s Gumbohatch vaccine and recommended the granting of a marketing authorization for the product. Gumbohatch protects chickens and embryonated chicken eggs against avian infectious bursal disease (IBD). (Animal Pharm)
- FRANCE Novogen announced it has acquired a hatchery Brittany, France. The new hatchery will house Grand Parent stock production, which is distributed worldwide, and Parent stock, which is distributed throughout the EMEA region (Europe, Middle East, Africa) and Asia. This new production facility supplements Novogen’s current installations in the US and Brazil. (company press release)
- SCOTLAND Roslin Technologies announced an agreement with Moredun Research Institute, Scotland’s Rural College and The Roslin Institute at the University of Edinburgh in Scotland to fund the commercial development of an Escherichia coli O157:H7 vaccine for cattle to prevent life-threatening illnesses in people. The experimental vaccine has been developed to limit E. coli O157:H7 shedding from, and transmission between, cattle. (Feedstuffs)
***********************************
ANIMAL HEALTH STOCK PRICE TRACKER
![]()
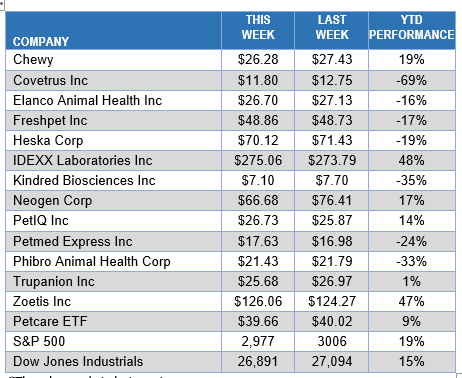
*Thursday market closing prices
***********************************
ANIMAL HEALTH NEWS
- US – ANIMAL DRUG APPROVAL The FDA released a draft Guidance for Industry, entitled “Eligibility Criteria for Expanded Conditional Approval of New Animal Drugs,” to assist animal drug sponsors and potential sponsors who may be interested in pursuing conditional approval to market animal drugs. Until recently, only new animal drugs intended for minor use in major species or for use in a minor species (MUMS) were eligible for conditional approval. In 2018, Congress amended section 571 of the Federal Food, Drug and Cosmetic Act (FD&C Act) to expand FDA’s authority to grant conditional approval to include certain animal drugs for use in major species (horses, dogs, cats, cattle, pigs, turkeys, and chickens) for diseases or conditions that would not be eligible for conditional approval under the MUMS provisions of the FD&C Act. For more information, go to https://www.fda.gov/animal-veterinary/cvm-updates/fda-issues-draft-guidance-expanded-conditional-approval-certain-animal-drugs?utm_campaign=9-26-2019-GFI261&utm_medium=email&utm_source=Eloqua. (FDA)
- US – ANTIMICROBIAL USE The FDA is accepting public comments through December 24, 2019 on draft “Guidance for Industry (GFI) #263” explaining the recommended process for voluntarily bringing remaining approved animal drugs containing antimicrobials of human medical importance (i.e., medically important) under the oversight of licensed veterinarians by changing the approved marketing status from over the counter (OTC) to prescription (Rx). For more information, go to https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-263-recommendations-sponsors-medically-important-antimicrobial-drugs-approved-use-animals. (FDA)
- US – The USDA’s Agricultural Research Service (ARS) announced plans to build three new state-of-the-art research facilities on one of its current bases in Kerrville, Texas. The project – valued at $54 million – will involve: the development of a new administration and laboratory building of approximately 50,000 square-feet; an 8,000 square-foot fly and tick research building; and an ancillary building space, which is expected to be 18,000 square-feet. Additionally, the ARS will construct new holding pens and other structures for livestock research. (Animal Pharm)
- US – VETERINARY SCHOOLS The Texas Tech University System hosted a groundbreaking ceremony for its upcoming School of Veterinary Medicine in Amarillo, Texas. It is estimated the school will cost about $90 million for facilities, but private donations have been raised that will aid in covering those costs, according to The Texas Tribune. The facility will include both a main site and a large-animal facility offsite in Amarillo. Tech still needs approval from the Texas Higher Education Coordinating Board and another third-party accreditor for its academic program. (KCBD.com)
************************************
BRAKKE CONSULTING VIEWPOINT
This is a busy, hectic time for many of you. You’re wrapping up the third quarter and working on budgets for next year. You’re trying to forecast the next three months and then hoping your budget doesn’t get changed too much once the full year results are in. And you’re trying to figure out what disruptions might change everything despite your plans.
One of those disruption you must be assessing is how veterinary products gets into the hands of pet owners. Chewy is one of those channel disruptors. The company saw a 60% increase in its initial $22/share stock price after its IPO on June 13th, but the stock has settled down and trades now in the $27/share range. It’s not profitable yet but revenue is growing fast – 43% year over year growth. That’s a big number.
But in their report, two bigger number caught my attention. They reported that the amount the company sells through “autoship” rose 49% and accounted for 69% of the quarter’s sales. In their S-1 filing, Chewy showed that once consumers start making purchases from them, they steadily increase purchases each year. And they are enhancing this stickiness by adding pharmacy and healthcare products. Two bigger numbers: they claim to have 3 million pet profiles on file and 12 million active customers…and they want to make the vet experience better.
All of us know we will see changes ahead in how pet owners buy products in the future, from pet food, to medications, pet care supplies to supplements. How fast strategies and tactics change from animal health companies and the veterinary industry remains to be seen. Maybe we’ll see them in next year’s budgets.
Bob Jones
**********************************
Trouble viewing this newsletter? You can view it online at www.BrakkeConsulting.com
This electronic newsletter is the sole property of Brakke Consulting.
Any use of the contents herein should be approved by and appropriately attributed to Brakke Consulting.
For more information about Brakke Consulting’s services and syndicated studies, visit our website at www.brakkeconsulting.com.
Brakke Consulting
806 Green Valley Rd
Suite 200
Greensboro, NC 27408
